It was the ultimate mystery protein of human cells — a tenant of the nucleus whose activities were unknown and whose shape was unlike that of any other protein. For Matthew Meyerson, MD, PhD, then an assistant professor, the challenge was direct and unambiguous: Figure out what it does.
Over the next 20 years, Meyerson, now director of Dana-Farber's Center for Cancer Genomics, and other scientists would not only unravel the role of the perplexing protein — called menin — but also place it at the center of two very different kinds of cancer. Their work led to the development of menin-targeting agents that today are showing promise in clinical trials involving adults with acute myeloid leukemia (AML), one of the hardest-to-treat forms of leukemia.
The story of research in menin and its relation to cancer — from early efforts to understand how the cell makes use of menin, to explorations of the cell machinery operated by menin, to the development and testing of agents that inhibit menin, to work on preventing resistance to such agents — weaves through Dana-Farber at every step.
What's My Line?
Meyerson recalls the quandary initially presented by menin. To determine a protein's function within a cell, researchers often make inferences from similarly shaped proteins. In menin's case, however, "there was no homology — structural likeness — to other proteins," Meyerson remarks. "It was a totally unique protein in the nucleus."
Researchers knew that an inherited condition known as multiple endocrine neoplasia type 1, which places people at risk for a variety of cancers, arose from a mutation in the gene responsible for menin, MEN1. From that and other findings, they determined that menin is a tumor-suppressor protein, standing guard over cell growth and division to slow them down, or even kill the cell, if they threaten to get out of hand.
Meyerson's goal was to determine how it accomplishes this. In a paper published in 2004, he and his associates reported their findings. Menin, it turns out, acts as a companion to a complex of other proteins, including one called MLL. The complex applies chemical units called methyl groups to histone proteins that hold DNA in place, a process called histone methylation. The effect is to switch on a set of genes that help rein in cell division.
Although menin itself doesn't attach methyl groups to histones, it enables the protein complex to do so. When menin isn't working properly because of a mutation in its gene, histone methylation is disrupted. As a result, some of the genes that rein in cell division do not get activated, and a barrier to runaway cell growth is lost.

Matthew Meyerson, MD, PhD, works to unlock the secrets of menin, a protein that may play a pivotal role in cancer treatment.
Unraveling the Paradox of Menin
Meyerson and others' research had established menin as a good and decent protein whose malfunction or absence could set the stage for cancer. That reputation seemed to be at risk when Meyerson and Michael Cleary, MD, MS, of Stanford University published a study in 2005.
The paper focused on a form of AML driven by an abnormality in a gene called KMT2A. The abnormality is known as a rearrangement, in which the gene becomes lodged in the wrong section of the genome, surrounded by unfamiliar DNA. The rearrangement gives rise to an abnormal "fusion" form of MLL that helps turn normal white blood cells into leukemia cells.
Meyerson and Cleary reported that in AML with rearranged KMT2A, menin can interact with the MLL fusion protein — and, in doing so, spur the development of leukemia. A protein whose sole purpose is to protect against cancer is, in this case, abetting the development of AML, a disease diagnosed in more than 20,000 people in the United States every year.
This seeming contradiction makes sense when one looks again at the liaison between menin and the protein complex containing MLL. In their relationship, the MLL complex is the doer, menin is the facilitator: The MLL complex methylates histones, and menin provides the impetus to do so. When MLL is corrupted by a mutation, menin remains a trusty sidekick, rousing MLL into action even if it sets the cell on a course for leukemia. In this way a normal, beneficial protein is duped into becoming an accomplice for cancer.

Picking Pockets
When scientists identify a mutant protein that drives cancer, the next step is often to find or create a small molecule that can hinder the protein's action. Block the protein, block the cancer process. By that standard, the mutant MLL protein in AML appeared to be an excellent target for a small molecule drug, but for one problem.
For a small molecule to be effective in a drug, it needs to wedge firmly into one of the nooks and crannies in a protein's surface. Unfortunately, the mutant MLL protein has few such landing spots, known colloquially as deep pockets.
Here, the work of Meyerson, Cleary, and others was pivotal. Scientists now knew that for mutant MLL to cause mischief, it needs an assist from menin. If MLL is a poor target for drugs, what about its indispensable partner? Would blocking menin so it couldn't cooperate with MLL be a way to render MLL harmless?
Menin, in turns out, has big, beautiful pockets for drug molecules. When researchers halted the production of menin in leukemia cells driven by MLL, the cells lost their cancerous qualities and began behaving more like normal, well-adjusted white blood cells.

Scott Armstrong, MD, PhD
Scott Armstrong, MD, PhD, senior vice president for drug discovery and chief research strategy officer at Dana-Farber, was quick to see the potential of a new approach to AML therapy.
"My lab had a longstanding interest in how gene rearrangements like those that create the MLL fusion protein lead to leukemia," Armstrong relates. "In 2006, we published a study identifying the specific set of genes controlled by the fusion protein. We began to look for ways to disrupt that protein's function — to turn the leukemia program off.
"Over time, thanks to the work of Matt [Meyerson] and others, it became clear that menin is critical for the function of the fusion protein. That touched off a major effort by pharmaceutical firms to develop drugs able to inhibit menin."
Wanted: Molecules to Block Menin
In the mid-2010s, Armstrong's laboratory began working with Syndax Pharmaceuticals to identify and test potential drug molecules that are active against menin. The lab team studied the effect of candidate molecules in leukemia cell lines and in animal models.

"Because we knew exactly which genes are switched on by the fusion protein to drive leukemia, we looked for evidence that those genes' activity was being reversed," Armstrong says.

A 3D illustration of acute myeloid leukemia cells.
In 2019, Armstrong and his colleagues published a paper showing that one such molecule not only worked well in animal models of human leukemia but shut down the exact set of genes driving the disease. Because it acted on those genes and no others, side effects were minimal.
These findings and others set the stage for the first clinical trial of a menin inhibitor in patients with acute leukemias harboring rearrangements of the KMT2A gene or mutations in another gene, NPM1. Dubbed the AUGMENT-101 trial, it tested an agent called revumenib — essentially the same compound that worked so well in Armstrong's 2019 paper — in 68 patients, nearly all of whom had been treated multiple times before for the disease.
The results, published last year, were impressive: 30% of the participants experienced a complete remission, a disappearance of all signs and symptoms of cancer. The findings were especially significant because, far from being rare forms of AML, those with KMT2A rearrangements or NPM1 mutations make up about 40% of all cases of the disease.
Also noteworthy, particularly in an early-phase trial, few participants had severe side effects.

Richard Stone, MD
"Seeing patients who were very advanced in their leukemias respond so dramatically to this agent and go into remission — in some cases, making them eligible for a stem cell transplant — has been very exciting," says Richard Stone, MD, Dana-Farber chief of staff and director of translational research in the Adult Leukemia Program, who led Dana-Farber's participation in the trial.
The results may have implications far beyond the treatment of AML. "The concept of using a drug to block an interaction between two proteins could be applicable to a wide range of cancers," Stone remarks. "Most targeted cancer drugs work by inhibiting an overactive enzyme, or protein. But many cancers require the kind of protein-protein interaction we see in in AML with KMT2A rearrangements. By inhibiting that interaction, we've shown it's possible to silence the genes that drive the disease."
Cat and Mouse
Menin inhibitors can put AML in check, but they can't always call checkmate. Armstrong's lab is now tackling the problem of drug resistance, in which AML wriggles free of revumenib and restarts its growth. The researchers have uncovered one way this occurs: A genetic mutation subtly alters the menin protein, preventing revumenib from binding to it firmly. This allows menin to mingle once more with the MLL fusion protein, sparking the leukemia process.
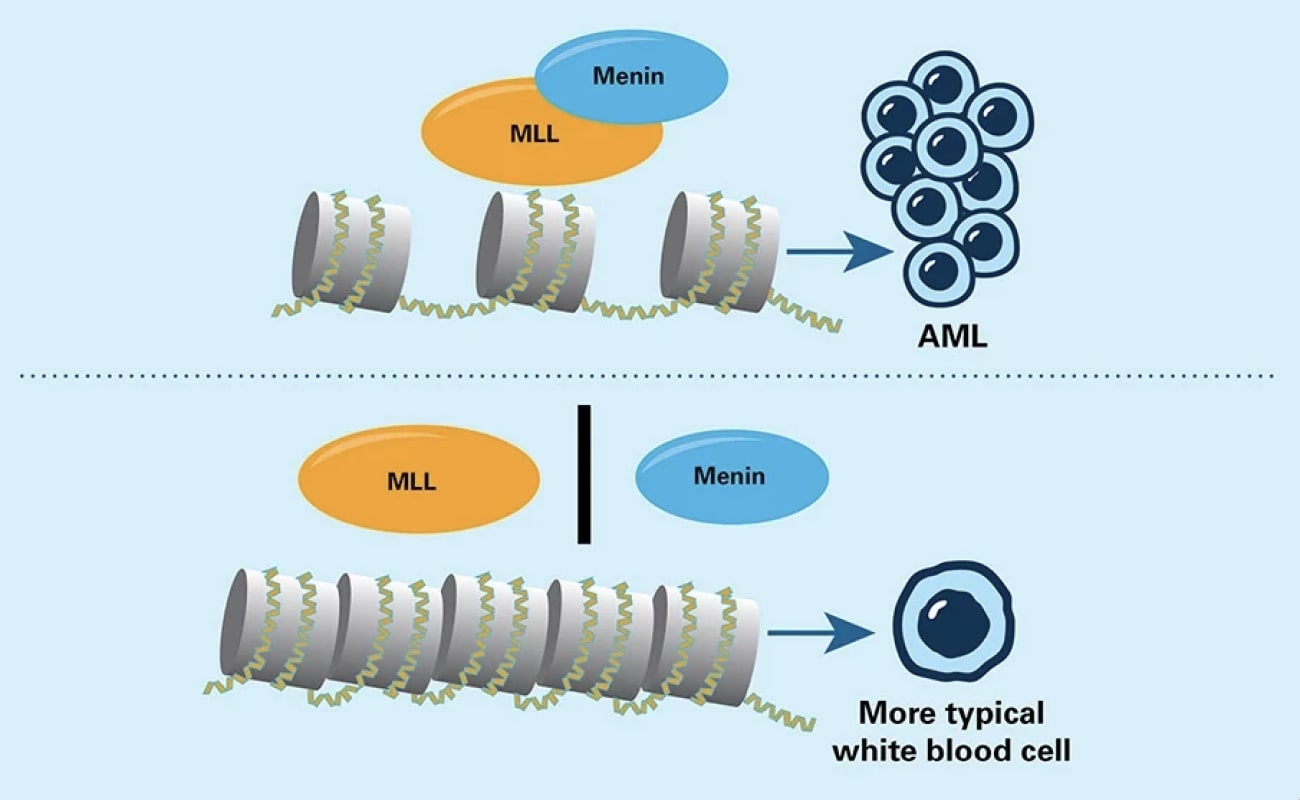
In some types of acute myeloid leukemia (AML) cells, the abnormal MLL protein joins with the menin protein to activate genes that promote leukemia. Drugs that keep MLL and menin apart hinder those genes from being switched on.
Two options for striking back suggest themselves. One is to develop drug molecules that deprive MLL of any place to bind onto menin. Another is to combine menin inhibitors with drugs that use a different mechanism for keeping MLL and menin apart.
While the development of resistance is frustrating, it also carries a certain irony: from scientists' perspective, its occurrence is evidence that they're on the right track in attacking the disease. "When a cancer goes to the trouble of developing a mutation to prevent a drug molecule from binding, it means the target protein is incredibly important to the cancer's survival," Armstrong comments.
And for researchers whose work over the past two decades has made possible drugs like revumenib, the agents' success is especially gratifying. "We began by just trying to understand what menin does," Meyerson remarks. "I'm delighted it's having such a positive impact for patients."