Recent News
March 14, 2017
Could Systemic Chemotherapy Hamper Success?
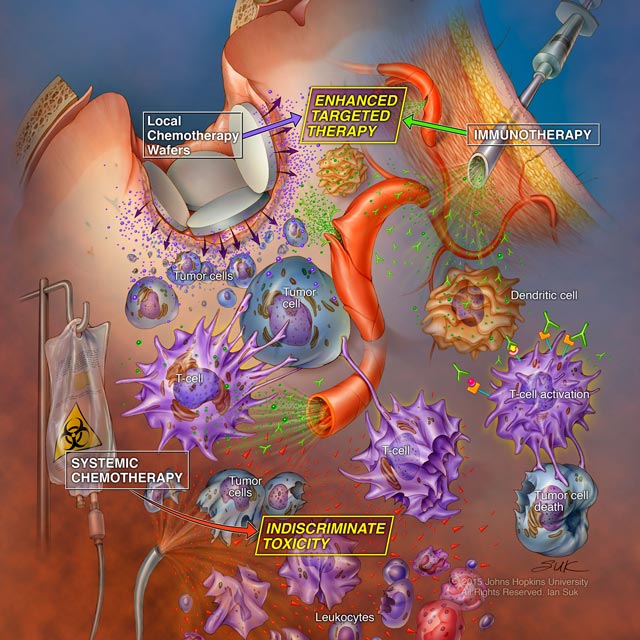
Illustration courtesy of Ian Suk, professor, Departments of Neurosurgery and Art as Applied to Medicine
A new study in animal models by Johns Hopkins researchers suggests that systemic chemotherapy appears to weaken the immune system so profoundly that immunotherapy can’t work to its fullest potential in fighting cancer. On the other hand, local chemotherapy appears to act in concert with immunotherapy, giving the best chance for long-term survival. The findings, published Dec. 21, 2016, in Science Translational Medicine, could change the course of cancer care.
The study focuses on checkpoint inhibitors, a class of immunotherapy drugs approved since 2014 for treating a variety of cancers, including advanced melanoma, head and neck, lung, kidney, Hodgkin lymphoma and bladder. Prognosis has historically been poor for many of these diseases, but the new drugs have provided long-term survival for up to 30 percent of patients in clinical trials.
“These drugs aren’t experimental treatments anymore. They’re FDA-approved treatments that work for certain types of cancers,” says Michael Lim, associate professor of neurosurgery and director of the Brain Tumor Immunotherapy Program at The Johns Hopkins Hospital and the Bloomberg~Kimmel Institute for Cancer Immunotherapy. “Where these compounds fall into our current algorithm of treatments is the next big question.”
Currently, Lim says, checkpoint inhibitors are offered when cancers continue to progress after patients receive the standard of care, including one or more chemotherapies. However, a notorious side effect of most chemotherapy drugs is marked damage to the immune system—a consequence that could significantly compromise an effective immune antitumor response.
Systemic vs. Local Chemotherapy
One potential way around this conundrum is to administer chemotherapy locally rather than systemically, which could help preserve immune function.
To test whether checkpoint inhibitors work better in combination with systemic or local chemotherapy, Lim’s research team administered the immunotherapy drug anti-PD-1 to a murine model of glioblastoma. They then dosed the animals with either systemic carmustine—a common chemotherapy for glioblastoma in human patients—three times weekly or implanted polymer wafers infused with the same drug locally at the tumor site.
Comparing blood sampled before the treatments and then at regular points afterward, the researchers found that the mice who received systemic chemotherapy had dramatically lower numbers of lymphocytes than those that received local chemotherapy. For example, those that received systemic chemotherapy had only about a third as many lymphocytes as those that received local chemotherapy two weeks after the end of treatment and still lagged behind four months later.
This dive in immunity appeared to significantly affect the utility of treatment. About 50 percent of mice that received combined systemic chemotherapy and immunotherapy were still alive after 100 days, compared to 80 percent of mice that received local chemotherapy and immunotherapy.
“Local chemotherapy and immunotherapy appeared to work synergistically, boosting the survival rate. But systemic chemotherapy didn’t offer any advantages and seemed to dampen the effects of immunotherapy,” Lim says.
Simulating patients with recurring disease, the researchers re-challenged long-surviving animals that got either treatment protocol with new tumors. All that received systemic chemotherapy and the checkpoint inhibitor died. But animals that received local chemotherapy and immunotherapy survived, becoming essentially immunized against their glioblastoma.
Variations on these experiments—administering chemotherapy before immunotherapy or using temozolomide, another chemotherapy agent commonly used to treat glioblastoma—showed the same trends. The researchers are currently investigating whether this phenomenon holds true for other chemotherapy drugs and other types of cancer. They are also planning a clinical trial to help determine the most effective way to incorporate checkpoint inhibitors into current treatments.
“Right now, the standard of care includes chemotherapy and radiation, which are both immunosuppressive. We may need to reconsider our strategy if we want to effectively incorporate immunotherapy,” says Lim. “It’s an exciting time, but we have a lot of work ahead of us.”
CUTTING-EDGE BRAIN TUMOR RESEARCH THAT TRANSLATES INTO PATIENT CARE
Johns Hopkins’ brain tumor research has an unparalleled history of introducing new forms of treatment for patients affected by malignant brain tumors, including the only two effective surgical treatments for malignant brain tumors approved by the FDA—carmustine and the GliaSite Radiation Therapy System.
Our leading role in the design and completion of multicenter clinical trials allows us to apply the most promising discoveries from the laboratory to patient care in an effective and expeditious fashion. At present, our most exciting areas of research include immunotherapy of brain tumors, adult stem cells and local delivery of a variety of new drug treatment options for brain tumors.
Glioblastoma
For newly diagnosed glioblastoma multiforme (GBM), we offer the following two studies. Patients are directed to one of these two studies based on their methylation status, which needs to be done on study.
NCT02617589: A randomized phase II open-label study of nivolumab versus temozolomide each in combination with radiation therapy in newly diagnosed adult subjects with unmethylated MGMT (tumorO-6-methylguanine DNA methyltransferase) glioblastoma
NCT02667587: A randomized phase II single-blind study of temozolomide plus radiation therapy combined with nivolumab or placebo in newly diagnosed adult subjects with MGMT-methylated (tumor O6-methylguanine DNA methyltransferase) glioblastoma
Recurrent GBM
NCT02766699: A phase I study to evaluate the safety, tolerability and immunogenicity of EGFR (Vectibix sequence)-targeted EDVs containing doxorubicin (EGFR(V)-EDV-Dox) in subjects with recurrent GBM
Meningioma
NCT02523014: A phase II trial of SMO/AKT/NF2 inhibitors in progressive meningiomas with SMO/AKT/NF2 mutations
Learn more at trials.johnshopkins.edu.
